As a curious marketer and lifelong learner, I engaged my friend, Google. Only to be sucked into a vortex, trying to get a better sense of what HEOR and RWE are and why marketers should care.
Did you know? HEOR has been around since the 1960s but is finally getting the limelight because of the value RWE brings.
Wondering, why now? I did.
According to Sara Alwardt, Vice President of Health Informatics and Health Economics and Outcomes Research of McKesson Specialty Health, the reasoning is twofold:
So what does this mean for marketers?
I put this overview together to create a baseline understanding of the discipline.
What is HEOR?
According to The Professional Society for Health Economics and Outcomes Research (ISPOR), HEOR is:
- The confluence of two bodies of work: Health economics and outcomes research, which, when combined, provide insights and evidence for stakeholders in the healthcare industry.
- The health economics (HE) part of the discipline measures the value of an intervention, such as a treatment or therapy.
- The outcomes research (OR) piece is a set of disciplines that generate insights and evidence to show the impact the intervention has/d on patients.
- Data and insights that can be used to help life sciences companies develop strategies and plans, perform landscape assessments and database analyses, map evidence, create economic models, gain payer insights, and support health technology assessments (HTAs).
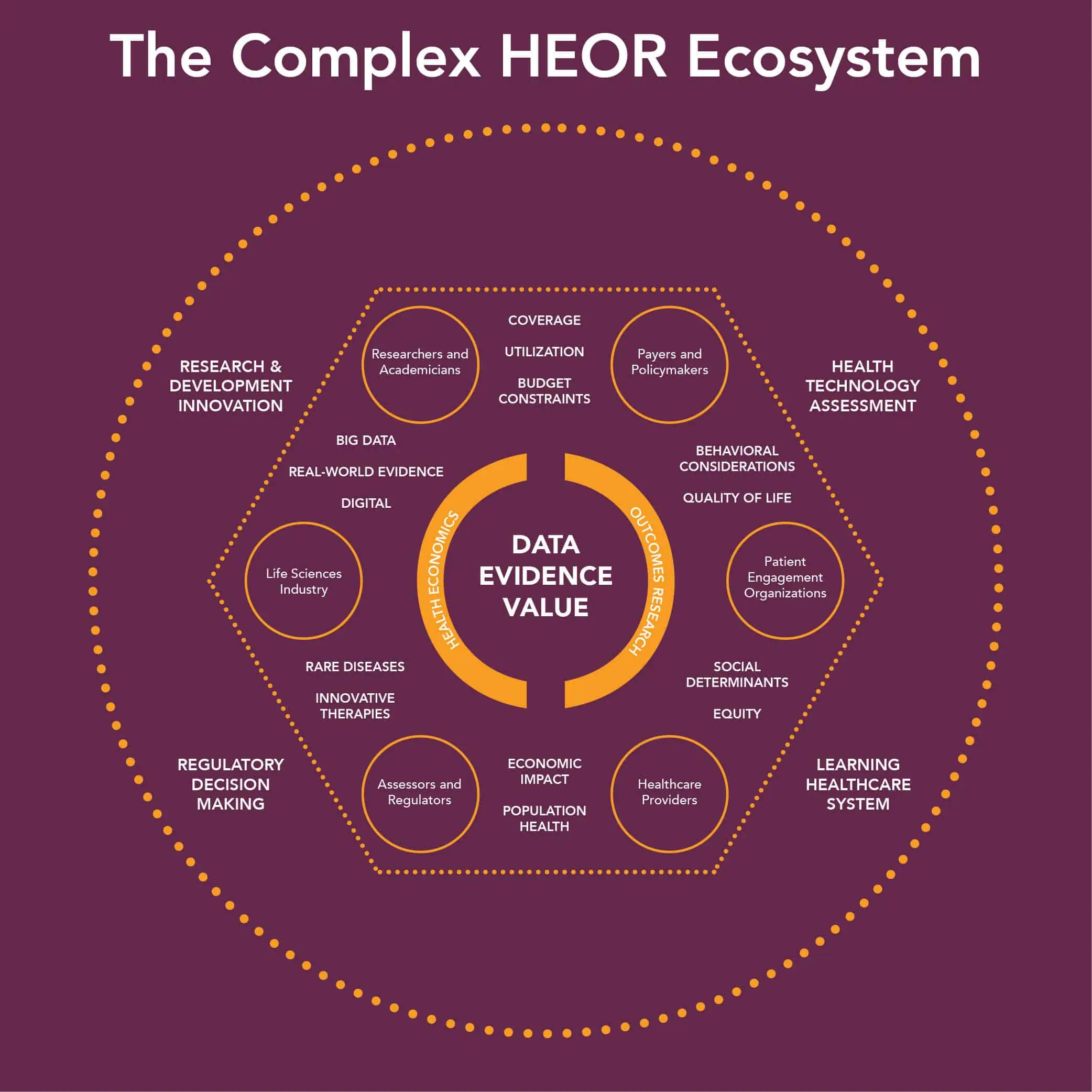
And that’s just the tip of the complexity iceberg. Review the diagram* to see all the areas HEOR touches:
* Source: ISPOR, showing The Complex HEOR Ecosystem
What is RWE?
Here’s an overview of the basics of RWE:
- According to the FDA, RWE “is the clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of RWD”.
- ISPOR includes RWE under the umbrella topic of HEOR.
- RWE is generated through traditional analytic and statistical methods, but machine learning and AI are providing more flexible ways of gathering insights along with predictive findings, faster.
- Using RWE in healthcare decision-making continues to be the number one trend according to ISPOR’s Top 10 HEOR Trends for 2022-20233. The FDA also supports this since the passing of the 21st Century Cares Act, placing focus on the use of RWD and RWE to aid in decision-making.
How is RWE used?
Here are a few industry use cases:
- RWE is used by life sciences companies both prospectively and retrospectively to generate evidence and provide answers to guide drug development, support regulatory approval, reimbursement, and market access.
- The results of RWE research studies influence the direction of drug development and commercialization. For example, it may save a pharmaceutical company millions of dollars by answering a clinical trial design question or may trigger that same company to pivot their strategy due to findings in the landscape assessment.
- RWE can also be used to support study design, augment clinical trials, and understand drug utilization, treatment patterns, and the burden of illness for example.
Additionally, HEOR and RWE are part of very salient topics in the healthcare industry:
- Health equity: helping to ensure clinical trials are more equitable.
- Patient voice: bringing the patient’s voice to the table, so to speak, by looking at what is happening in the real world.
- Drug pricing: enhancing the dialogue around drug pricing by involving payers throughout the lifecycle.
I believe HEOR and RWE are and will continue to be incredibly powerful in advancing global healthcare to safely accelerate access to life-saving drugs for everyone, as we are all patients.
Our life sciences marketing agency works with companies offering HEOR and RWE expertise, research, consulting, and analytics by developing marketing plans and content marketing strategies that speak to your buyers and influencers.
Are you interested in working with a marketing agency that speaks fluent healthcare, life sciences, and biotech? We can’t wait to meet you.
Additional sources:
- https://www.mckesson.com/Blog/Using-Real-World-Evidence-to-Improve-Biopharmaceutical-Drug-Outcomes/
- https://www.ispor.org/heor-resources/about-heor
- https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence
- https://www.ispor.org/heor-resources/about-heor/top-10-heor-trends